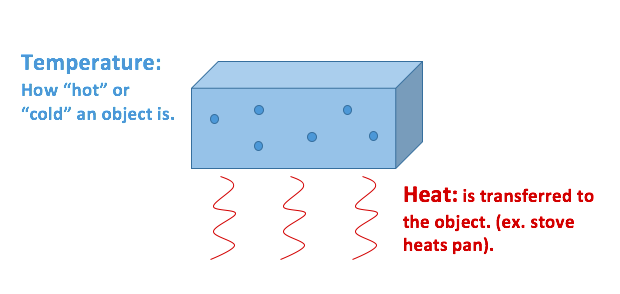
 Heat and temperature are a closely related topic, and as such, the difference between the two can be a bit confusing. The core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy.
Heat and temperature are a closely related topic, and as such, the difference between the two can be a bit confusing. The core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. The main difference between heat and temperature is heat is the overall energy of the molecular motion, whereas temperature is the average energy of the molecular motion.
The main difference between heat and temperature is heat is the overall energy of the molecular motion, whereas temperature is the average energy of the molecular motion.